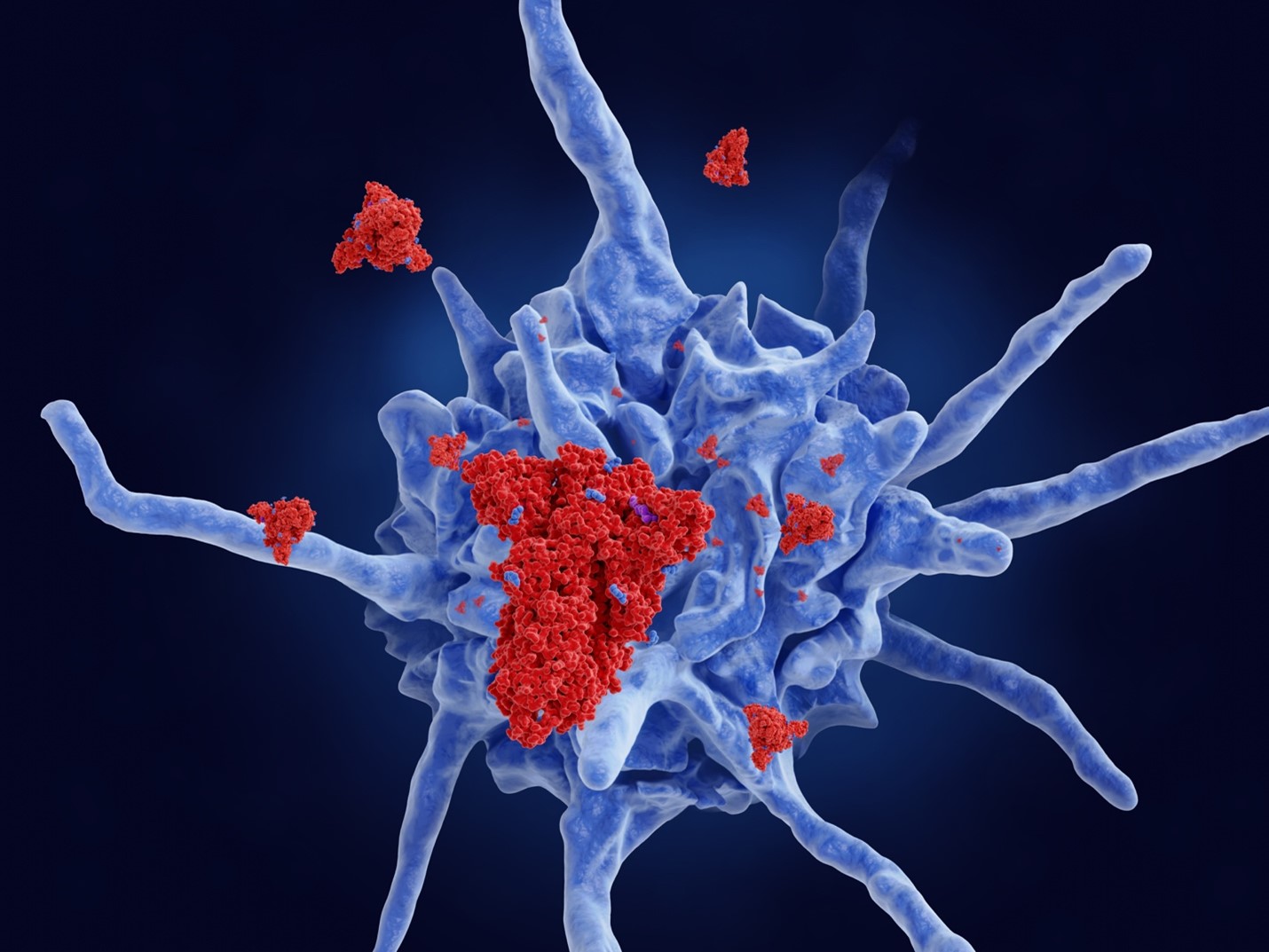
The SARS-Cov-2 Spike protein has been attributed to many peculiar symptoms of COVID-19, like blood clotting and tissue damage. Using the cutting-edge cryo-electron tomographic technique, we directly observed SARS-Cov2 Spike proteins acting on the platelet surface. Our finding explains why SARS-Cov-2 can significantly jeopardize blood clotting compared to other coronaviruses.
SARS-Cov-2 is a respiratory virus responsible for COVID-19 infections that can be severe or even fatal. Peculiar to SARS-Cov-2, compared to other coronaviruses, is its tendency to lead to blood clotting defects, which are involved in the major causes of severe COVID-19 complications (like microthrombosis, strokes or multi-organ failure). What exactly leads to these coagulopathies is subject to discussions. Indirect immune reactions, direct platelet hyperactivation or an effect of the SARS-Cov-2 spike protein itself are all possible scenarios. However, no direct evidence to pinpoint and visualize the mechanism of platelet clotting caused by SARS-Cov-2 has been available.
In our study, we extracted platelets, a component responsible for blood clotting, from blood of healthy human donors and mixed them with SARS-Cov-2 spike protein. We then analyzed the change of the platelet activity using live imaging and cellular cryo-electron tomography (cryo-ET). Platelets are known to adhere to wounded tissue surfaces and convert their discoidal form to a starfish-like shape (i.e. activation). To avoid uncontrolled blood clotting, the activation of platelets is normally tightly controlled and only happens when the body surveillance system detects a scar. Specific coagulation signals trigger a response to repair the wound. Interestingly, in the presence of SAR-Cov-2 spike protein, platelets started stretching the shape from their typical discoidal shape without the coagulation signals. We then realized that this process of the spike protein-driven platelet shape change can be reversed, and platelets were able to return to their normal discoidal shape. This indicates that the SARS-Cov-2 spike protein primes platelets for activation. In some cases, it leads to a point of no return for the activation of platelets, resulting in coagulation.
In situ Cryo-ET is a new emerging technique that facilitates the direct observation of cells or tissue allowing to identify the activity states of individual proteins or other molecules inside cells. We used the cryo-ET approach to obtain a direct in-depth view into the cause of platelet deformation. Strikingly, we found that SARS-Cov-2 spike proteins densely decorated the surface of platelets, triggering a local change of the skeletal organization inside platelets. Bundles of skeletal filaments called actin were aligned to push the platelets from the inside to deform them. This micro-structure, called filopodia, is known as an initiation step in platelet activation. Actin filaments are connected to a signal receptor called integrin on the platelet surface. Integrin is the most abundantly expressed receptor on the platelet surface, acting as an antenna to receive signals to initiate platelet activation. Thus, our observation points to a scenario that the SARS-Cov-2 spike protein hijacks the pathway of integrin-mediated platelet activation. The spike protein is recognized by this platelet antenna, and then, integrin sends an activation signal to the platelet so that the platelet starts deforming for its activation. Of interesting note, the SARS-Cov-2 spike protein contains an ‘RGD’ amino acid docking motif, which is also known as a common ligand motif recognized by integrin. The ‘RGD’ is an evolutionary acquisition of SARS-Cov-2, which does not exist in other human-infecting coronaviruses, explaining the specific coagulation symptoms characteristic to COVID-19 patients.
One important functional question is, why the SARS-Cov-2 spike protein only activates platelets occasionally, why the effect is mostly reversible, further, why the coagulation would not be common among all COVID19 patients? To answer these questions, we were prompted to test the direct interactions between SARS-Cov-2 spike protein and platelet-expressed integrin receptors, by purifying receptor proteins and bringing them into an isolated test environment (in vitro). Finally, we identified that the SARS-Cov-2 spike protein preferably interacts with a minor type of platelet integrin avb3 instead of the major class expressed in platelets, integrin aIIBb3. This finding explains why blood clotting can be significantly jeopardized by SARS-Cov-2 but severe cases are rather rare events, while most of the COVID-19 patients do not experience obvious blood clotting pathologies. Our findings will help to find targets to treat severe cases of COVID-19 infections.
Original Article:
Kuhn, C. C., Basnet, N., Bodakuntla, S., Alvarez-Brecht, P., Nichols, S., Martinez-Sanchez, A., Agostini, L., Soh, Y.-M., Takagi, J., Biertümpfel, C., & Mizuno, N. (2023). Direct Cryo-ET observation of platelet deformation induced by SARS-CoV-2 spike protein. Nature Communications, 14(1), 620. https://doi.org/10.1038/s41467-023-36279-5
 Health & Physiology
Health & Physiology